Creatine loading protocols have been remarkably stable amidst other advancements in sports performance nutrition. The University of Guelph’s Human Performance and Health Research Laboratory is testing a new topical creatine supplement that challenges several mainstays of those protocols.
Graduate students Alanna Whinton and Rachel Aubry are conducting this leg of the research, which is part of a multi-centre study. They have split their investigation into two studies. The first phase examined the acute effects of the creatine cream, while the second phase focused on chronic application. The second phase also measured arterial stiffening, to determine any impact of resistance exercise with chronic creatine cream application. Both phases measured average power, peak power and fatigue rate using a 1080 Quantum connected to a standard leg extension machine.
Participants in both studies performed 5 sets of 15 maximal leg extensions to establish a baseline. They then returned one week later to repeat the 5 x 15 session.
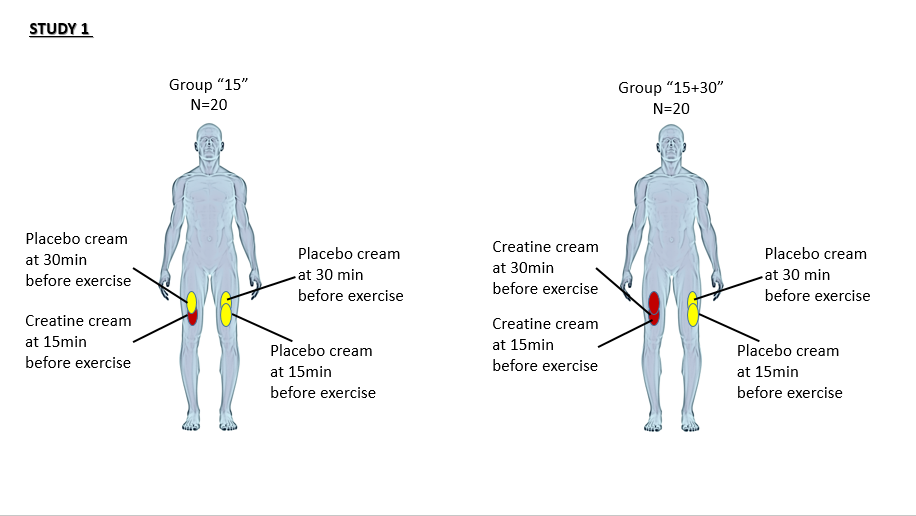
In Study 1, Whinton and Aubry divided the participants into two groups. Participants in both groups received a placebo cream on one leg 15 minutes and 30 minutes before exercise, and the creatine cream on the other leg 15 minutes before exercise. The “15+30” group, though, received an additional application of the cream 30 minutes before exercise.
Study 2 looked at the effect of the creatine after a week of usage. Participants in the experimental group received either an oral creatine supplement or an oral placebo, along with two bottles of cream: one creatine, one placebo. The control group received nothing at all. The experimental group ingested their supplement three times a day and applied the cream once per day (always the same treatment to the same leg).
The oral and topical supplements were given to the participants double-blind. They did not know to which leg they were applying the creatine or the placebo. This design results in each participant having one “control leg” and one “experimental leg” against the oral supplement condition (placebo or creatine), in addition to the full control.
The efficacy of the supplement and the broader question of acute, localized creatine usage are only part of Whinton and Aubry’s investigation. While they used well-established protocols for testing an ergogenic aid, they developed a novel testing and measurement tool to perform the procedure.
Instead of using an isokinetic dynanometer, they connected a 1080 Quantum to a standard leg extensor machine. Female participants kick against 5 kilograms of resistance while men kick against 8 kilograms. This facilitates maximal power output without the participants feeling like they are kicking against a load. Whinton and Aubry set the speed on the 1080 Quantum to .5 meters / second. They determined during through their validity and reliability study (see the poster here) that .5 m/s on the 1080 Quantum correlated to 180 degrees / second on an isokinetic dynamometer.
Whinton and Aubry’s unique equipment creates a greater level of versatility at a significantly reduced cost compared to standard equipment. A 1080 Quantum can be connected in a similar fashion to nearly any piece of resistance equipment.
More validity studies will reveal the correlations between “gold standard” equipment and 1080-based equipment. This will enable researchers and practitioners to test an increasing number of movements by adding a single new piece of equipment to their existing infrastructure.
[av_button_big label=’RIG Academy drives American football ambitions in Sweden’ description_pos=’below’ link=’manually,http://1080motion.com/dream-big-work-smart-american-football-sweden/’ link_target=” icon_select=’no’ icon=’ue800′ font=’entypo-fontello’ custom_font=’#ffffff’ color=’theme-color’ custom_bg=’#444444′ color_hover=’theme-color-subtle’ custom_bg_hover=’#444444′ av_uid=’av-2ptf5z’][/av_button_big]
The Guelph researchers caution that the machines should not be used interchangeably. But continual demonstrations of repeatability and reliability open the door to using the 1080 Quantum in more human performance studies.






























































